DehydraTECHTM-powered semaglutide achieved these benefits in a human pilot study compared to Rybelsus®:
- Sustained higher levels of semaglutide in blood;
- Faster achievement of peak drug delivery; and
- Reduced side effects.
KELOWNA, BC / November 27, 2023 / Lexaria Bioscience Corp. (NASDAQ:LEXX)(NASDAQ:LEXXW) (the “Company” or “Lexaria”), a global innovator in drug delivery platforms announces positive interim results from a human pilot study (the “Study“) currently underway evaluating DehydraTECHTM technology for the oral delivery of the glucagon-like peptide-1 (“GLP-1“) drug semaglutide available commercially in the branded product Rybelsus®.
The Study is being performed by a prominent university research center comparing a single 7 mg semaglutide dose of a Rybelsus tablet (“Control“) to a matching dose from Rybelsus that had been compound formulated in capsule form using DehydraTECH processing technology enhancements (“DehydraTECH GLP-1“).
Blood Levels of Semaglutide
Blood was examined by a third-party laboratory using a validated bioassay 18 times over the first 10 hours of the Study, and once again 24 hours after the dose was administered. Subjects were supervised and monitored in the Study site over the entire 10-hour duration post dosing, and then allowed to depart, resume normal activities and return to the Study site the following day for performance of the 24-hour evaluation time point. The first post-baseline blood was sampled 20 minutes after oral administration and, at that point in time, the DehydraTECH GLP-1 blood semaglutide level was ~125% higher than that of the Control. At each of the 19 blood sample time points, the DehydraTECH GLP-1 blood semaglutide levels were higher than the Control levels. Furthermore, the DehydraTECH GLP-1 peak was achieved faster at 120 minutes (as compared to 160 minutes for the Control) and with a 16% higher blood semaglutide level than the Control. Of note, the blood semaglutide levels achieved with the Control were proportional to those achieved by other researchers in similar single dose cross-over Rybelsus development informing studies, further suggesting that the blood semaglutide level gains evidenced by the DehydraTECH GLP-1 formulation were notably distinct relative to the commercial product.
Blood Levels of Semaglutide
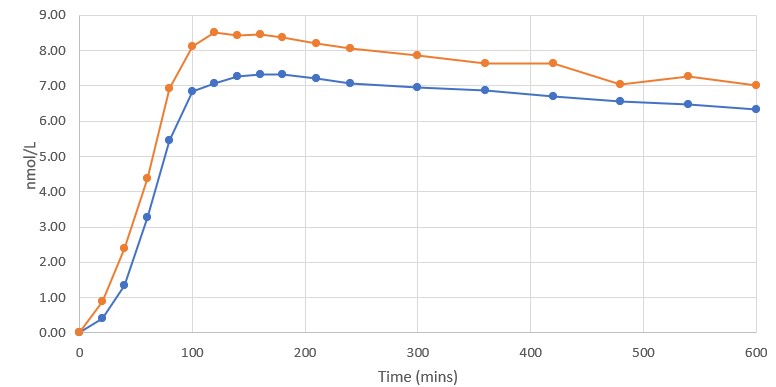
Rybelsus (blue) 7mg (n=3) DehydraTECH (orange) GLP-1 7mg (n=4)
Interestingly, even 24 hours after dose administration, the DehydraTECH GLP-1 blood semaglutide levels were still approximately 25% higher than the Control levels (Note – only the first 10 hours post dosing is shown in the graph above). Overall, the total semaglutide drug quantity based on the area under the curve or “AUC” delivered by the two interventions over the course of the 24 hour Study monitoring period was approximately 15% higher in the case of the DehydraTECH-GLP-1 versus that of the Control.
Side Effects
Two of the three Control subjects and zero of the four DehydraTECH GLP-1 subjects reported experiencing moderate nausea. All three Control subject reported experiencing mild nausea at both the 2-hour and 10-hour timepoints whereas only one DehydraTECH GLP-1 subject reported mild nausea and only at the single, 2-hour timepoint.
Ongoing and Future Work
This Study is only meant to provide early-stage indicative information to Lexaria about the possibility of enhancing the pharmacokinetic (“PK”) and pharmacodynamic performance of orally delivered GLP-1 drugs to assist in guiding the Lexaria team in additional investigations.
This Study is not complete and additional results will be reported, likely in two tranches: Lexaria has also collected blood glucose data and expects to release those interim results imminently. Then, once the cross-over Study visit as described below has been conducted, final results from the Study should be available in late December or early January. Given the small sample size of this Study, it was not sufficiently powered for statistical significance analysis, which will be a key part of any expanded studies with DehydraTECH GLP-1 undertaken in the future.
Parallel to Lexaria’s 2021 optimization program with DehydraTECH-processed cannabidiol (“CBD”), where the formulation utilized in the animal study DIAB-A22-1 demonstrated 364% higher (p=0.0002) PK performance than Lexaria’s original DehydraTECH-CBD formulations, we expect to create several different DehydraTECH GLP-1 formulations to explore delivery and performance optimization of semaglutide. As seen with our past DehydraTECH-CBD advancements, Lexaria will endeavor to similarly improve performance of future DehydraTECH-GLP-1 formulations.
Lexaria has demonstrated in many previous R&D programs, including five human clinical studies, that DehydraTECH can greatly improve the PK performance of many orally administered drugs into the bloodstream. Like many of these agents, GLP-1 drugs also exhibit very poor oral bioavailability (as little as 0.8%) without the use of absorption enhancement technology, such as Lexaria’s DehydraTECH. As noted above, DIAB-A22-1 evidenced DehydraTECH-CBD lowering body weight over a sustained dosing period by 7%, and also lowered blood sugar and triglyceride levels.
Design has already begun of a comprehensive animal PK and efficacy modelling study program to pursue these goals using different DehydraTECH compositions and different GLP-1 drug molecules. The Company cannot know in advance whether future formulations will perform better or worse than that which was used in the current Study. We are currently planning to begin the DehydraTECH-GLP-1 animal study during Q1, 2024.
Separately, Lexaria is also exploring the possibility of studying DehydraTECH-GLP-1 in a multi-week human clinical trial to examine both diabetes-related control (in part via reduced blood sugar levels) as well as weight loss following treatment. If design parameters allow, we intend to test, separately, DehydraTECH-GLP-1, as well as a combination of DehydraTECH-CBD with DehydraTECH GLP-1, based on the noteworthy effects of DehydraTECH-CBD evidenced in animal study DIAB-A22-1. Before proceeding with this human evaluation, we hope to have the animal formulation PK and efficacy modelling test results so that we can use the best-performing formulation in the multi-week human clinical trial. Thus, all things being favourable, we expect the multi-week human clinical trial could start near the end of Q2 or sometime in Q3, 2024.
Execution of the above-described animal study and multi-week human clinical trial will be contingent on raising additional capital necessary to fund doing so. Through these expanded studies, Lexaria hopes to achieve superior PK performance using DehydraTECH-powered GLP-1 drugs which could enable drug delivery via oral capsule at lower costs than current injectables, with reduced side effects and enhanced health benefits. Ultimately, Lexaria’s goal in doing so will be to demonstrate advancements worthy of commercial product development and potential pharmaceutical industry strategic partnering interest, for superior performing oral GLP-1 drugs as more viable and attractive alternatives to the injectable format.
About the Study
The Study was performed to provide an early-stage indication of whether DehydraTECH processing could improve oral drug delivery characteristics of the GLP-1 drug semaglutide sold as Rybelsus. A single semaglutide dose of 7 mg of the Rybelsus Control was compared to the matching dose of the DehydraTECH GLP-1, swallowed by each subject after an overnight fasting period together with a 50 mL glass of water. The DehydraTECH GLP-1 formulation used in this Study was compound formulated strictly for research purposes. Seven healthy subjects were dosed, four of whom received the DehydraTECH GLP-1, and three of whom received the Control. These seven subjects are expected to return to the Study site in December to be dosed a second time in the reverse order following the “cross-over” design of this Study to ensure that all seven subjects will have been treated with both the Control and DehydraTECH GLP-1 treatments over the course of the two visits.
About GLP-1 Drugs.
Rybelsus (semaglutide) is the only GLP-1 drug approved by the FDA for oral dosing to treat diabetes and weight loss. The FDA has also approved semaglutide marketed as Ozempic® and Wegovy®, administered by injection, to treat diabetes and weight loss. All three of these drugs are owned and manufactured by Novo Nordisk.
GLP-1 drugs have recently been approved by the FDA for type two diabetes and weight loss management. Weight loss of between 10 pounds to 33 pounds, or more, has been widely reported. One 68-week study of 667 people reported an average loss of 15% of body weight.
Anecdotal commentary also suggests that some patients are experiencing reduced cravings for alcohol, nicotine and opioids while taking GLP-1 drugs. Other trials are examining their effects on heart disease and even dementia in part because of evidence that GLP-1 drugs may reduce the build-up of the proteins amyloid and tau in the brain, thought to be partly responsible for Alzheimer’s disease.
Side effects of GLP-1 drugs vary but can include nausea, vomiting, diarrhea and more. A small number of GLP-1 drugs have already been tested or approved in oral format but some studies have reported worse side effects with the oral form. The drugs are also being investigated for their relationship to bone density, muscle loss and more. Because of potential serious side effects, it may be beneficial to treat patients with lower oral doses of the drugs, something that Lexaria’s DehydraTECH technology may enable if it can improve the PK performance of GLP-1 drugs through oral capsules.
Because GLP-1 drugs have experienced FDA approvals as recently as 2021 and 2022, and because the health benefits of this drug class are still being discovered and understood, the potential market size is unknown. Published reports are widely estimating $100 billion in sales per year, by 2030. At least one analyst from Guggenheim Partners published a note on September 12, 2023 in which he explained how “the total addressable market for these so-called incretin drugs could balloon to $150 billion to $200 billion.”
About DehydraTECH
DehydraTECH is a patented drug delivery formulation and processing platform technology Lexaria has developed and is investigating for a variety of beneficial molecules. DehydraTECH is designed to improve the way active molecules enter the bloodstream upon oral ingestion. DehydraTECH has also demonstrated enhanced delivery of certain active molecules into brain tissue, which Lexaria believes to be of particular importance for centrally active compounds. Lexaria has also developed DehydraTECH formulations for other applications demonstrating superior bio-absorption when administered intraorally and topically.
About Lexaria Bioscience Corp.
Lexaria Bioscience Corp.’s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream through oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids, antiviral drugs, PDE5 inhibitors and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 37 patents granted and many patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as “anticipate,” “if,” “believe,” “plan,” “estimate,” “expect,” “intend,” “may,” “could,” “should,” “will,” and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the Company relating to the Company’s ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company’s best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company’s ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company’s public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. The Company provides links to third-party websites only as a courtesy to readers and disclaims any responsibility for the thoroughness, accuracy or timeliness of information at third-party websites. There is no assurance that any of Lexaria’s postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements or links to third-party websites contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
George Jurcic – Head of Investor Relations
[email protected]
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.
View source version on accesswire.com:
https://www.accesswire.com/809947/lexarias-technology-improves-the-oral-performance-of-the-rybelsusr-branded-glp-1-drug-semaglutide-in-human-pilot-study