DehydraTECH-CBD offers distinctive mechanistic benefits related to its growing therapeutic utility
KELOWNA, BC / May 23, 2023 / Lexaria Bioscience Corp. (NASDAQ:LEXX)(NASDAQ:LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms is pleased to announce additional findings from last year's human clinical study HYPER-H21-4 ("the Study") demonstrating significant reductions in several pro-inflammatory biomarkers known to be linked to cardiovascular disease ("CVD") and a host of other conditions.
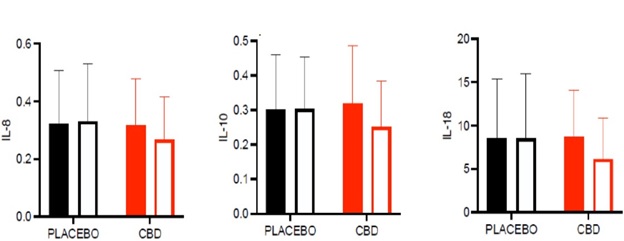
After five weeks of treatment with the patented DehydraTECH-processed cannabidiol ("CBD") capsule formulation, blood-plasma levels of interleukin ("IL") 8, 10, and 18 were reduced by ~19%, ~27%, and ~43%, respectively. Those persons receiving five weeks of placebo experienced no significant changes in their IL levels as shown with the black bars in the graph below. The differences evidenced relative to baseline and/or placebo with pro-inflammatory biomarkers IL 8, 10 and 18 were statistically significant (p<0.05).
Blood Plasma Levels (pg/mL) of Pro-Inflammatory Biomarkers IL-8, IL-10 and IL-18
"There is some pre-clinical evidence for the anti-inflammatory actions of CBD, but this is likely the most convincing evidence in humans that I have ever seen," said Dr. Philip Ainslie, Cardiovascular Advisor to Lexaria and Lead Investigator of Study HYPER-H21-4. "The bigger picture is that inflammation is the key basis of atherosclerosis, and several pro-inflammatory agents have been examined as potential mediators of the biochemical pathways of lesion formation. Other ‘common' diseases or disorders associated with chronic inflammation include fatty liver disease; Type 1 & 2 diabetes mellitus; inflammatory bowel disease; asthma; lung diseases chronic kidney disease; rheumatoid arthritis and obesity. Part of the reason why many of these diseases lead to cardiovascular disease is via chronic inflammation."
There is clear scientific evidence for the involvement of IL-8 in the establishment and preservation of the inflammatory microenvironment of the vascular wall in instances of CVD. In cardiovascular risk estimation, several reports have indicated that increased serum levels of IL-8 are correlated with an increased risk of CVD or acute cardiovascular events.
IL-10 and IL-18 are also considered anti-atherosclerotic cytokines with evidence for IL-8 and IL-18 perhaps being even stronger in CVD. In addition, IL-10 levels are known to increase along with the reduction of kidney function; and higher serum IL-10 levels have been associated with the risk of cardiovascular events during follow-up. Similarly, IL-18 is an independent predictor of cardiovascular events in patients with metabolic syndrome. In large population-based studies, circulating IL-18 is prospectively and independently associated with CVD risk.
Lexaria previously announced that the primary efficacy and safety objectives of the Study were successfully achieved, with resting blood pressure significantly reduced in hypertensive patients, and sustained over the full 5-weeks of dosing with zero serious adverse events being reported throughout the Study. Lexaria is aware of only a handful of other published research studies, mostly in young, healthy and normotensive volunteers, that have investigated whether a sustained decrease in resting blood pressure is possible following multiple weeks of oral CBD dosing; none of which have been successful in achieving this.
In addition, Lexaria also previously announced data from the Study revealing potentially a unique mechanistic benefit upon cardiovascular regulation via catestatin modulation with DehydraTECH-CBD treatment that has not previously been demonstrated with testing of CBD for blood pressure reduction to its knowledge. This finding together with today's announcement of improvements in pro-inflammatory biomarker levels related to cardiovascular and related disease states further strengthens the case for DehydraTECH-CBD offering distinctive mechanistic benefits related to its growing therapeutic utility. These reductions in inflammation also provide an mechanistic additional pathway by which DehydraTECH-CBD may act to lower blood pressure.
The Food and Drug Administration ("FDA") has laid out clear guidelines for sponsors who seek to develop new anti-hypertensive drugs, specifically defining the need for medications that offer complementary modes of action. Lexaria believes that its latest results, detailed below and already peer-reviewed and published in the respected journal, "Biomedicine and Pharmacotherapy", may support DehydraTECH-CBD qualification within these FDA guidelines.
DehydraTECH's Relationship to the Hypertension Market
Over 100 million adult Americans have high blood pressure, but only one in four of those have the condition under control. Many patients stop taking their medications because of troublesome side effects: some diuretics can cause excessive urination, beta blockers can cause erectile dysfunction, calcium-channel blockers can cause leg swelling, and ACE inhibitors can lead to persistent cough. Lexaria believes that its DehydraTECH-CBD may introduce a more tolerable anti-hypertensive treatment option that may be used alone or in combination with other medications, to reduce blood pressure with fewer discouraging and unwanted side effects.
There is a clear medical and market need for alternative anti-hypertensive therapies, especially in the case of patients with so-called resistant hypertension who fail to adequately control their high blood pressure with combinations of existing medications. Of note, the decreases in blood pressure evidenced in the Study were similar in both those hypertensive patients on standard of care blood pressure medications and those who were untreated for their hypertension upon Study entry. Therefore, Lexaria's findings indicate that Lexaria's DehydraTECH-CBD has the potential to offer complementary and additive blood pressure reduction benefits on top of any degree of improvements the standard of care medications provided. This additive improvement of DehydraTECH-CBD as an adjunct therapy, perhaps related to its pronounced effectiveness in modulating catestatin and pro-inflammatory cytokine levels, could become a significant value enhancer should it eventually enter the marketplace as an approved hypertension treatment.
Study HYPER-H21-4 together with four previous human clinical studies in hypertension Lexaria conducted from 2018 through 2022 are integral to successful filing and review of its upcoming Phase 1b investigational New Drug ("IND") application to the U.S. Food and Drug Administration ("FDA") for purposes of its planned U.S. study HYPER-H23-1. Lexaria's five foundational human clinical studies to-date in this area have been carried out in an aggregate total of 134 healthy and hypertensive persons and have evidenced significant reductions in resting blood pressure over both acute and multi-week dosing regimens while producing zero serious adverse events.
Additional endpoint analyses from HYPER-H21-4 as described in the complete study protocol are still underway and any relevant material findings will be reported upon as these findings become available.
Study HYPER-H23-1 is entitled ‘A Phase 1b Randomized, Double-Blind, Placebo-Controlled Study of the Safety, Pharmacokinetics, and Pharmacodynamics of DehydraTECH-CBD in Subjects with Stage 1 or Stage 2 Hypertension'. The primary objective of the study will be to evaluate safety and tolerability in hypertensive patients, and secondary objectives will include efficacy evaluation in reducing blood pressure together with detailed pharmacokinetic testing. Lexaria anticipates filing the IND this summer with hoped-for FDA authorization within 60 days thereafter.
About DehydraTECH-CBD
DehydraTECH-CBD is a unique CBD formulation Lexaria has developed and is optimizing based on its patented and proprietary DehydraTECH drug delivery technology. DehydraTECH is designed to improve the way active molecules enter the bloodstream upon oral ingestion. DehydraTECH has also demonstrated enhanced delivery of certain active molecules including CBD into brain tissue, which Lexaria believes to be of particular importance for the effectiveness of its DehydraTECH-CBD specifically against hypertension because of the significant influence of central mediation upon blood pressure. Lexaria has also developed DehydraTECH-CBD formulations for other applications demonstrating superior bio-absorption when administered intraorally and topically.
About Lexaria Bioscience Corp.
Lexaria Bioscience Corp.'s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream through oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids, antiviral drugs, PDE5 inhibitors and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 30 patents granted and many patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the company relating the Company's ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company's ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. The Company provides links to third-party websites only as a courtesy to readers and disclaims any responsibility for the thoroughness, accuracy or timeliness of information at third-party websites. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements or links to third-party websites contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
George Jurcic - Head of Investor Relations
[email protected]
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.
View source version on accesswire.com:
https://www.accesswire.com/756166/Lexaria-Discovers-that-DehydraTECH-CBD-Treatment-in-Hypertension-Study-HYPER-H21-4-Resulted-in-Reduction-in-Pro-Inflammatory-Biomarkers