- DehydraTECH delivers equivalent quantity of oral THC three times faster: 15 minutes vs. 45 minutes
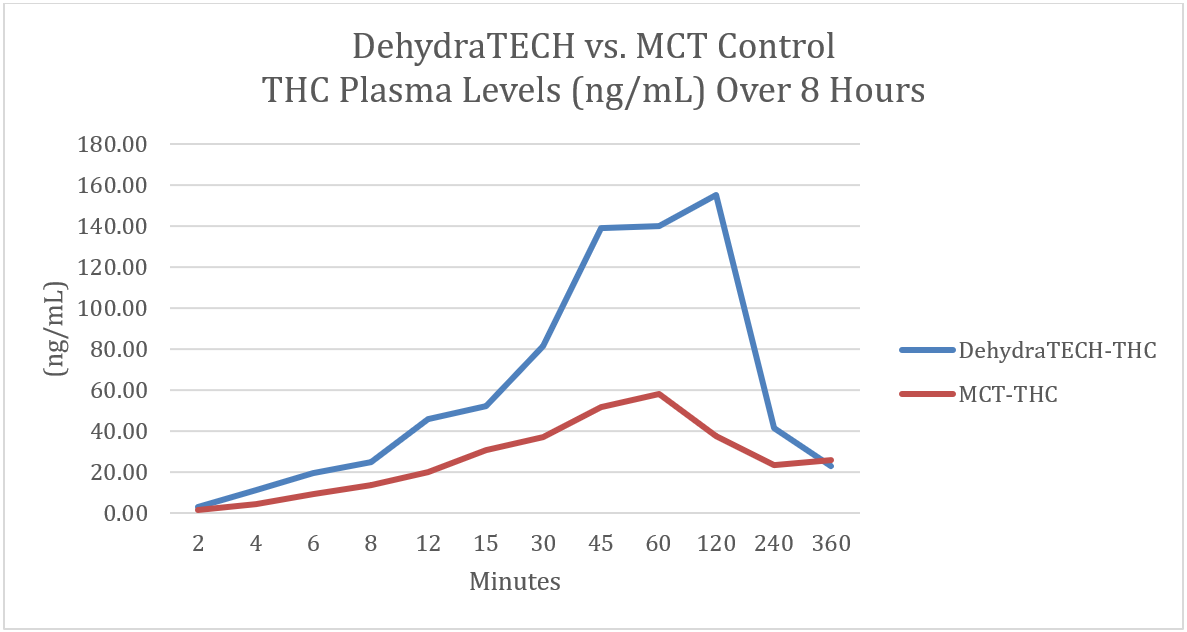
KELOWNA, BC / October 13, 2021 / Lexaria Bioscience Corp. (NASDAQ:LEXX)(NASDAQ:LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms is pleased to announce that its recent oral tetrahydrocannabinol ("THC") absorption study THC-A21-1 revealed that DehydraTECHTM-THC delivered via oral ingestion required only 15 minutes to deliver THC levels in blood plasma comparable to levels achieved at 45 minutes with concentration-matched controls.
During the study DehydraTECH-THC delivered more THC into bloodstream than the industry standard medium chain triglyceride ("MCT" or "coconut oil") based control formulation from the 2-minute mark onwards, then dropped rapidly to the same level as the MCT control by the 6-hour mark.
According to Harvard Medical School, the most common use for medical cannabis in the US is for the control of pain. Chronic pain is associated with nerve disorders and multiple sclerosis, and users are frequently cited as responding to a cannabis treatment program, without the highly addictive or sedating effects of opiates. Lexaria's investigation of enhanced delivery characteristics of THC utilizing DehydraTECH technology is focused on medical applications.
Key pharmacokinetic ("PK") findings from the study are tabulated below demonstrating statistically significant improvements in peak and total THC delivery (i.e., maximum concentration or Cmax and total area under the curve up to the point of the last measurement or AUClast respectively):
DehydraTECH-THC Cmax* % Improvement (ng/mL) | MCT Control THC (ng/mL) | DehydraTECH-THCAUClast** % Improvement (hr ng/mL) | MCT (Coconut Oil) Control THC (hr ng/mL) |
178.6 ± 81.1 110.9% (p=0.026) | 84.7 ± 43.9 | 539.2 ± 190.9 96.5% (p=0.013) | 274.4 ± 106.9 |
"The cannabis industry continues to use outdated formulations and processes that ignore the needs of modern THC users," said Chris Bunka, CEO of Lexaria Bioscience Corp. "THC users today include recreational, medicinal and pharmaceutical users, all of whom need technology that doesn't rely on harmful delivery methods such as smoking but still provides rapid onset and high bioavailability which common oral formats do not offer. Our study findings demonstrated rapid delivery, increased overall THC delivery, and higher brain tissue delivery; all of which is consistent with the wants and needs of THC customers."
DehydraTECH processing continues to prove its ability to deliver drugs more effectively not just into the bloodstream, but also into brain tissue. Only two time-points were selected to collect brain tissue absorption samples, meaning blood concentration levels likely had peaked much earlier, but DehydraTECH-THC brain tissue levels were effectively twice as high as the MCT controls, mirroring what was seen with the blood level readings achieved during the study:
Brain Absorption | DehydraTECH THC Improvement % (ng/g) | Coconut-Oil Control THC (ng/g) |
8-Hours | 121.0 ± 92.6 103.0% | 59.6 ± 20.5 |
24-Hours | 14.4 ± 10.3 108.7% | 6.9 ± 5.2 |
About Study THC-A21-1
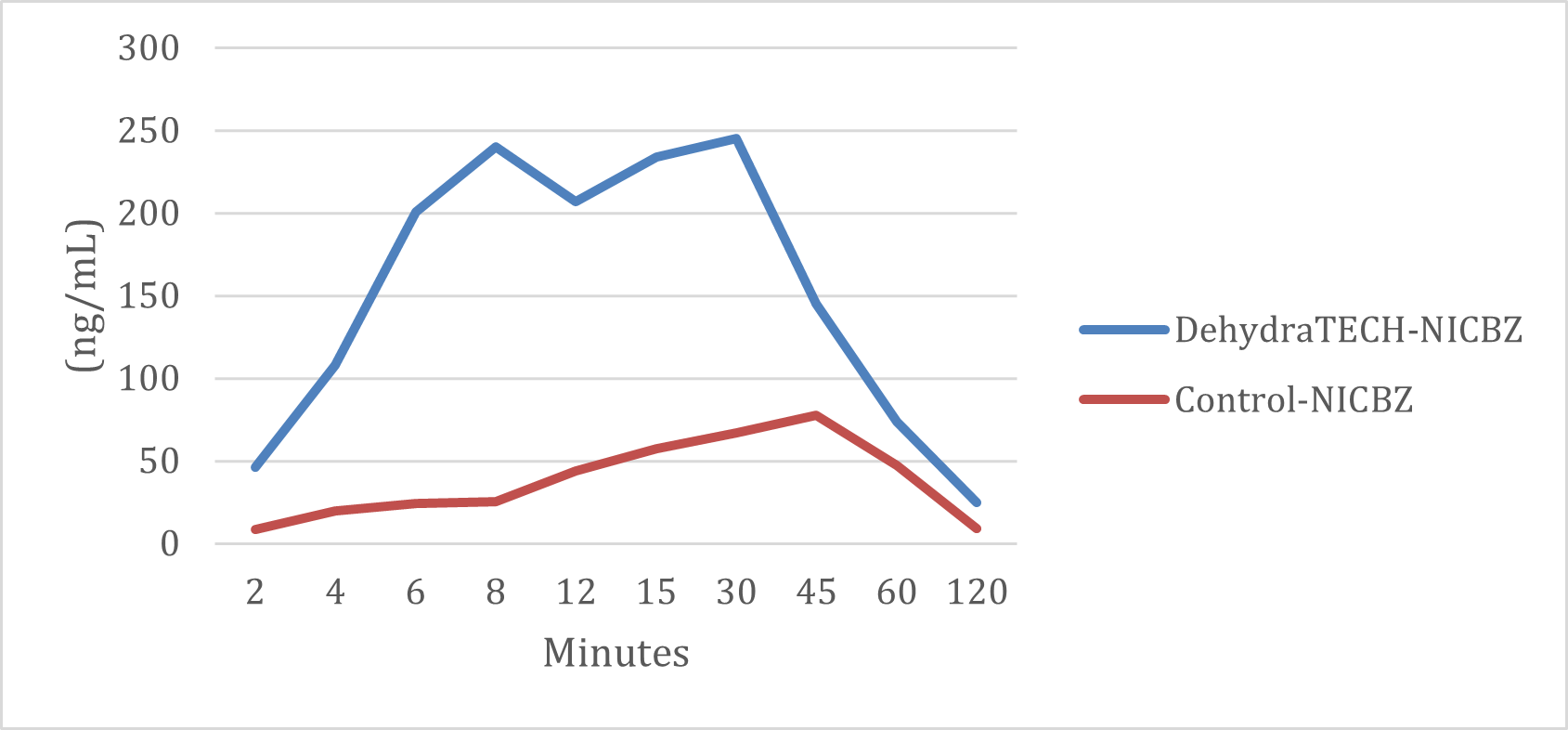
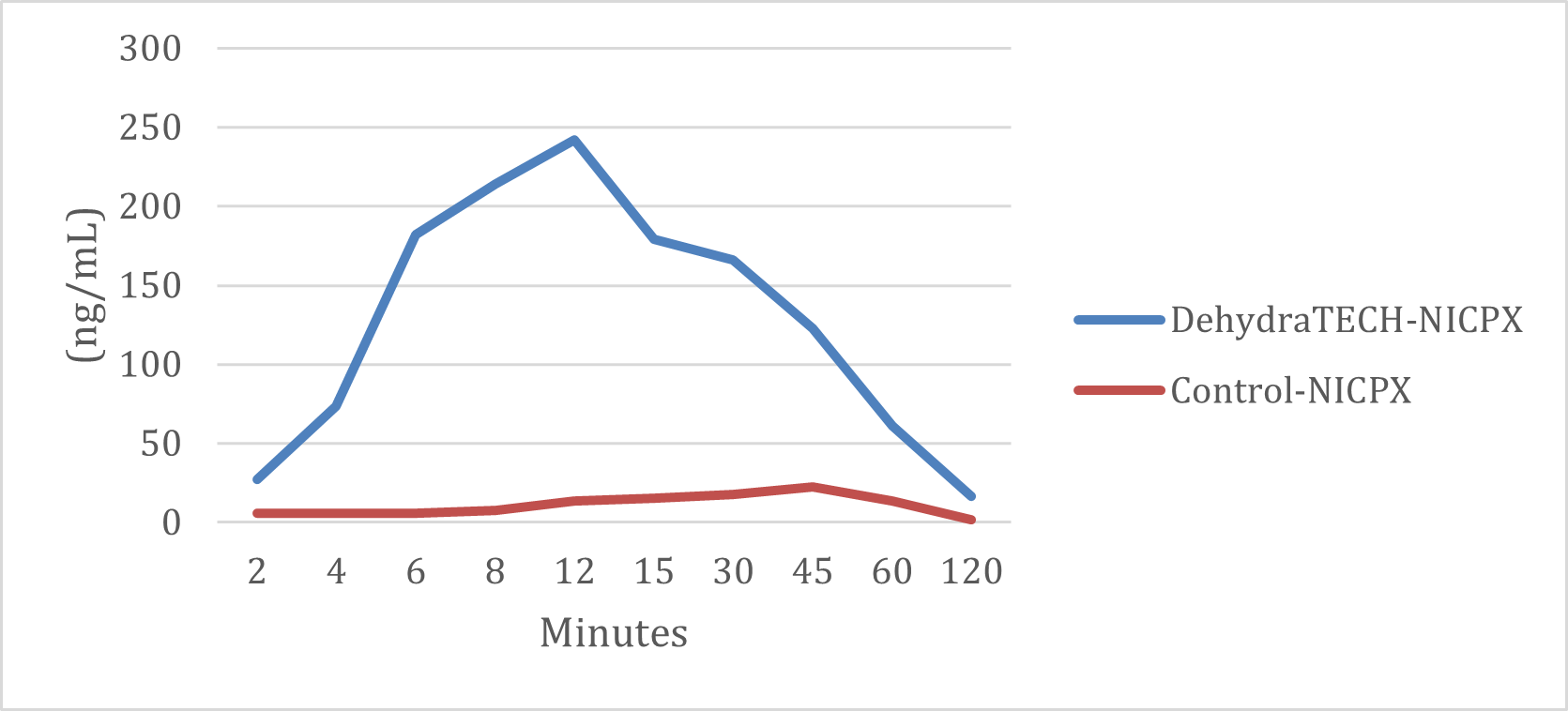
Study THC-A21-1 was performed by a leading, independent testing laboratory. Blood samples for 20 male Sprague Dawley rats (two groups of 10) are represented in the graph above taken at intervals of 2, 4, 6, 8, 12, 15, 30, 45, and 60 minutes, and at 2, 4 and 6 hours. Brain tissues were collected at 8 and 24 hours.
About Lexaria Bioscience Corp.
Lexaria Bioscience Corp.'s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream by promoting more effective oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids and nicotine by up to 5-10x, reduce time of onset from 1 - 2 hours to minutes, and mask unwanted tastes; and is also being evaluated for orally administered anti-viral drugs, non-steroidal anti-inflammatory drugs (NSAIDs), and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 21 patents granted and over 50 patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the company relating the Company's ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company's ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
[email protected]
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.
View source version on accesswire.com:
https://www.accesswire.com/667925/Lexarias-Technology-Proven-to-Deliver-Oral-THC-More-Effectively