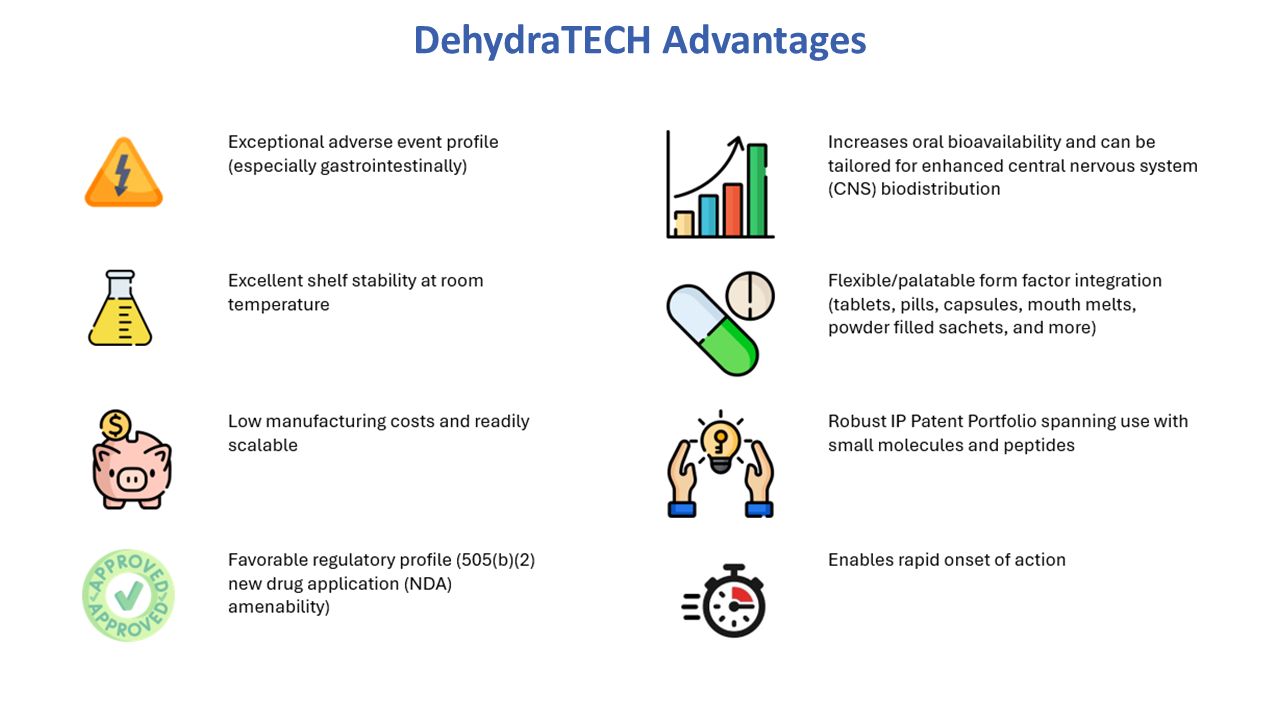
Lexaria Bioscience Corp’s (NASDAQ:LEXX) proprietary drug delivery-enabling technology, DehydraTECH™, improves the absorption of active pharmaceutical ingredients (APIs) into the bloodstream, allowing greater drug effectiveness often with reduced side effects.
DehydraTECH is a platform technology that can be applied to many different APIs and other bioactive substances, spanning both small and large molecule therapeutics, in oral formats including tablets, capsules, oral suspensions and more.