DehydraTECH appears to work with a second GLP-1 drug - liraglutide
DehydraTECH appears to be working with semaglutide both with and without SNAC technology
DehydraTECH-CBD is showing strong apparent performance relative to GLP-1
Dosing in the final four study arms has now begun
KELOWNA, BC / July 17, 2024 / Lexaria Bioscience Corp. (Nasdaq:LEXX, LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms announces that interim results from the first four weeks of dosing in its ongoing diabetes animal study WEIGHT-A24-1 (the "Study") have been received and have already produced several noteworthy findings.
Unlimited food and water has been provided to the animals for the entire duration of the Study. During the initial acclimation phase of 34 days before the beginning of dosing, the animals gained 10.9% body weight on average. During the subsequent 28 days of DehydraTECH-processed drug therapy dosing, all test articles showed either a stark decrease in the rate of body weight gain or, in select cases, the beginning of body weight reduction. DehydraTECH-CBD formulation 3 ("CBD3") and DehydraTECH-liraglutide have produced the largest weight loss results thus far of -1.50% and -1.58%, respectively. Of note, only in Study arms A, B, C, and E did any individual animals lose 5.0% or more body weight during these first 28 days of treatment. An additional 56 days of dosing remains across all treatment groups.
Animal Weights (grams)
DehydraTECH Groups | Arrival in Lab | End of Acclimation Period | % Change to End of Acclimation | End of 28 Days of Dosing | % Change to Day 28 |
A: CBD1 | 386.0 | 427.9 | +10.9% | 432.6 | +0.96% |
B: CBD2 | 360.6 | 394.6 | +9.4% | 393.3 | -0.59% |
C: CBD3 | 375.5 | 416.0 | +10.8% | 408.8 | -1.50% |
D: CBD4 | 377.2 | 431.2 | +14.3% | 431.7 | +0.32% |
E: Rybelsus1 | 365.1 | 394.9 | +8.2% | 394.6 | +0.07% |
F: Rybelsus2 | 364.5 | 406.2 | +11.4% | 409.1 | +0.72% |
G: Semaglutide | 352.7 | 394.2 | +11.8% | 394.8 | +0.16% |
H: Liraglutide | 354.6 | 392.2 | +10.6% | 385.7 | -1.58% |
Average | 367.0 | 407.1 | +10.9% | 406.3 | -0.18% |
Notes
-Weights shown are average of six animals per group.
- Groups A through D were varying DehydraTECH-CBD compositions
- Groups E and F were reformulated Rybelsus® compositions including DehydraTECH and the sodium salcaprozate ("SNAC") technology
- Groups G and H used pure GLP-1 drugs (semaglutide and liraglutide respectively) without SNAC inclusion
This is the first time that DehydraTECH processing was applied to the GLP-1 drug liraglutide, and it is encouraging to witness its relative outperformance. Although liraglutide was administered as an oral DehydraTECH-enabled dose in this Study, it is currently only sold and prescribed as a subcutaneous injection (see below for more context). Liraglutide is currently available commercially by Novo Nordisk® sold under the brand names Victoza® and Saxenda®, but the related patents have begun to expire and open the door to new competitors. For example, Teva Pharmaceutical Industries Ltd® recently launched the first generic injectable version of this GLP-1 drug.
Study arm G is the first time that pure semaglutide was tested with DehydraTECH processing without the SNAC technology found within the Rybelsus® tablets. It is of interest that it has, so far, apparently performed nearly identically in body weight gain attenuation to Study arm E which is the best performing DehydraTECH-processed reformulated Rybelsus® composition that includes the SNAC technology.
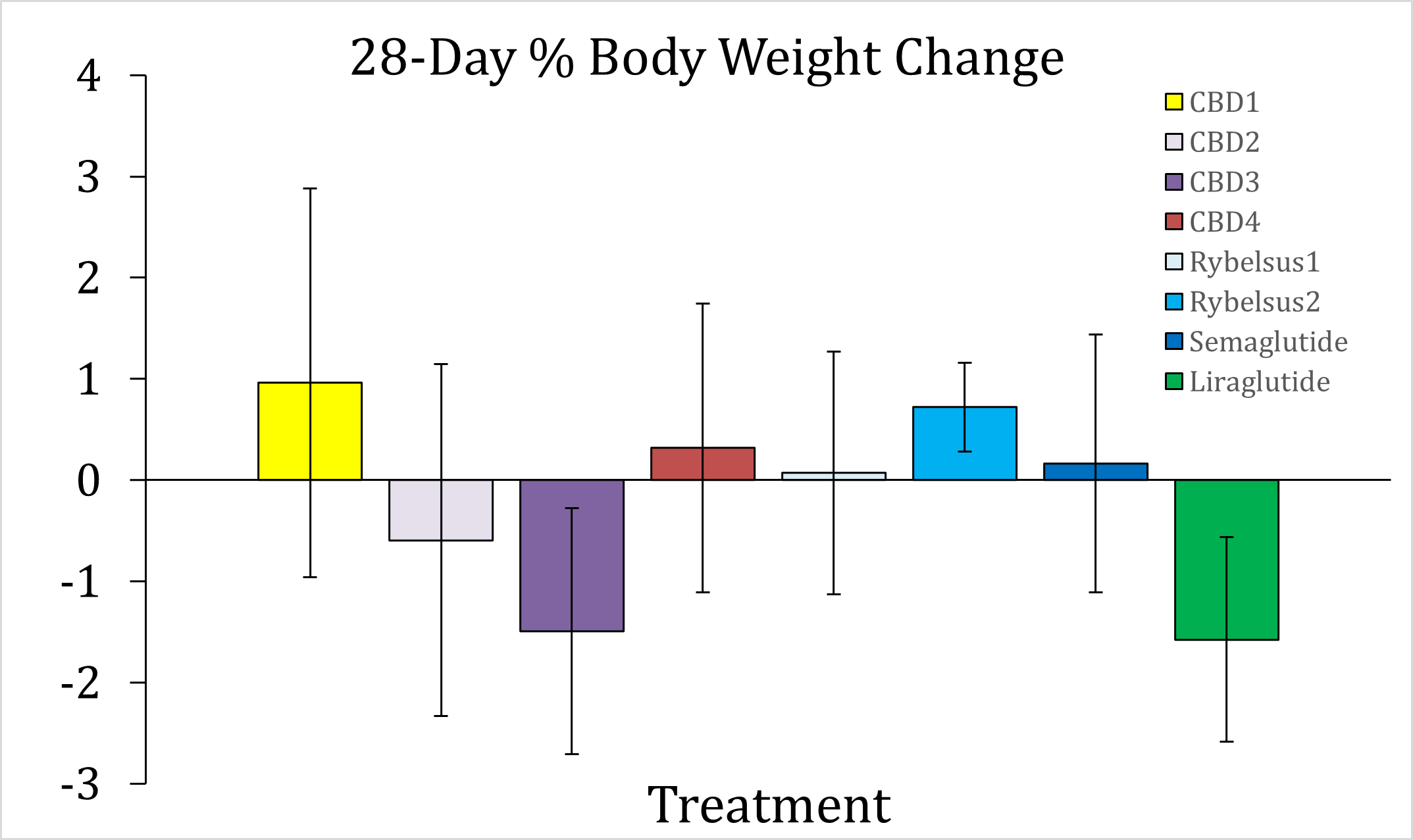
Another important objective has now been reached in evaluating the results of the first 28 days of dosing among the first eight Study arms that, together, constitute Cohort 1 of the Study: Performance superiority has been observed among the various formulations, allowing the Study team to select the best performers for inclusion in the subsequent Cohort 2 Study work. Dosing in those final four Study arms that constitute Cohort 2 of the Study has begun and is expected to be completed in mid-October.
Those final four Study arms include a positive control arm (commercially available Rybelsus®) and a placebo arm, as well as a combined DehydraTECH-semaglutide with DehydraTECH-CBD arm (Groups C + E), and a combined DehydraTECH-liraglutide with DehydraTECH-CBD arm (Groups C + H).
In all cases and as per design in an exploratory, directionally informative Study of this nature, animal populations were not intended to be large enough to necessarily evidence statistical significance, and readers are reminded that these data are indicative only of apparent trends that have not been analysed statistically. Blood glucose data for the first four weeks of dosing has also been received, is being processed, and will be released soon. Pharmacokinetic ("PK") data for the first four weeks has not arrived yet and will be released when available.
Brain absorption data can only be collected at the end of the 12-week study and will also be released when available. Readers are encouraged to review our May 17, 2024 press release that provides a thorough explanation of the comprehensive Study design and rationale, including why possible enhancement of brain absorption is believed to be of potential significance.
About Liraglutide
Liraglutide is owned by Novo Nordisk® and sold under the Victoza® and Saxena® brands which generated $1.6 billion in combined revenue in 2022. Liraglutide is currently administered only by injection, after attempts to utilize the SNAC technology found in semaglutide (and sold as the oral tablet Rybelsus®) failed to deliver sufficient performance to enable an oral form factor.
Previous investigators reported that "the absorption enhancing action of SNAC is thought to be highly dependent on the specific agent it is enhancing, which means that carefully tailored co-formulation is required rather than co-administration. The structure of liraglutide (a structurally distinct analog of GLP-1RA) was found to be unfavorable for co-formulation with SNAC on account of its stronger membrane-binding properties, which reduced transcellular passage, as well as its greater tendency to oligomerize, which countered the monomerizing effects of SNAC. In a preclinical study, plasma exposure was significantly higher for semaglutide than liraglutide after oral dosing with SNAC."
About the Study
Study WEIGHT-A24-1 is underway using diabetic, pre-conditioned Zucker rats. Each arm of the Study is expected to be dosed for a 12-week period following the initial acclimation period. During the Study, over 1,500 blood plasma samples will be collected from the total rat population of 72 animals for purposes of detailed PK drug delivery analyses. Body weight and blood glucose readings were taken prior to Study start continuing at regular intervals during and at conclusion of the dosing period. Upon completion of the Study, brain tissue will be analysed to help determine whether DehydraTECH processing results in higher brain absorption than non-DehydraTECH arms, as Lexaria has evidenced numerous times in previous similar animal studies. The Study will also include a comprehensive battery of liver and kidney function testing and blood chemistry analyses. LC-MS/MS and other techniques will be used to analyse samples.
About Lexaria Bioscience Corp. & DehydraTECH
DehydraTECH™ is Lexaria's patented drug delivery formulation and processing platform technology which improves the way active pharmaceutical ingredients (APIs) enter the bloodstream through oral delivery. Since 2016, Lexaria has developed and investigated DehydraTECH with a variety of beneficial molecules in oral and topical formats. DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption and has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier, which Lexaria believes to be of particular importance for centrally active compounds. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 46 patents granted and many patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the company relating the Company's ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company's ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. The Company provides links to third-party websites only as a courtesy to readers and disclaims any responsibility for the thoroughness, accuracy or timeliness of information at third-party websites. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements or links to third-party websites contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
George Jurcic - Head of Investor Relations
[email protected]
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.
View the original press release on accesswire.com