KELOWNA, BC / January 27, 2022 / Lexaria Bioscience Corp. (NASDAQ:LEXX)(NASDAQ:LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms, is pleased to provide an annual letter from CEO, Chris Bunka and a thorough strategic update to all stakeholders.
Dear Shareholders,
Will anyone ever forget 2021? If a global pandemic and unprecedented weather events don't resonate, then perhaps the tectonic changes in politics or social involvement in investing epitomized with GameStop might register. Against this constantly shifting backdrop, Lexaria faced challenges and delivered impressive results in many areas.
First, it may be useful to state Lexaria's key area of focus: improving our understanding of the capabilities of the ground-breaking DehydraTECHTM drug-delivery technology to enhance our abilities to commercialize and profit from this technology platform. Our applied R&D programs are designed to reduce future risks of both commercial and regulatory failure by identifying weaknesses as early as possible; and to enhance the likelihood of future commercial and regulatory success by thoroughly understanding strengths and capabilities, also as early as possible. As our data sets expand, they provide answers to many of the questions we expect to face from potential commercial partners, or from regulators, as we seek to advance those commercial pursuits.
DehydraTECH is not an EVOLUTION of existing technology - it is a REVOLUTIONARY new drug delivery platform. Expecting entire industries to change overnight to adopt a revolutionary new process is not realistic: it takes time, evidence, and a lot of positive results to overcome industry inertia. We have long been hopeful that, before the end of 2022, we will have built sufficient data to effect meaningful industry and/or regulatory progress and sustainable increases in valuation. Is 2022 the year that Lexaria begins to soar?
Shareholder/Market Achievements
As has been true for many years, I continue to be the largest shareholder of Lexaria - although just barely! We have a number of individual and institutional shareholders who have bought sizeable positions since our Nasdaq debut and we welcome you all.
In the following discussion and details about shareholders, some supportive explanation is required. NOBO shareholders are "Non Objecting Beneficial Owners": these are those of you who have permitted your identity to be known to us. OBO shareholders are "Objecting Beneficial Owners" and we, as a company, do not have access to your identity through the normal data suppliers. For OBO shareholders, we only have summary data of how many shareholders object to having their identity known, and collectively, how many shares they own together.
Our NOBO shareholder list currently covers 9,773 shareholders collectively owning 4.39 million shares and we have updates to much of that data, every day. We can and do follow trends and try to ensure that we are satisfying your needs.
Since listing on Nasdaq, we have witnessed an unusual phenomenon, namely the substantial increase in new shareholders who hold very small securities positions. We currently have over 600 shareholders who own 1 single share each; over 3,300 shareholders who own 10 shares or less; and an amazing 7,200 shareholders who own 100 shares or less.
At the other end of the scale, we have relatively few shareholders with large positions. We only have about 50 shareholders who own 10,000 shares or more; over 100 shareholders who own 5,000 shares or more; and about 650 shareholders who own 1,000 shares or more. Because of the small numbers of shareholders with large positions, it is easy for us to track changes even on large volume days.
In January of 2021, each of our 8,352 NOBO shareholders owned an average of 142 shares. By July of 2021, our 9,594 NOBO shareholders owned an average of 389 shares each. And in December 2021, our 9,773 NOBO shareholders owned an average of 449 shares each.
As 2021 progressed, our number of shareholders has increased, and the average number of shares owned per shareholder has increased by 216%. These are positive trends showing accumulation and general approval of Lexaria's business plans.
The institutional shareholders who participated in our January 2021 financing have, as expected, mostly sold their positions: their business is in the provision of capital, not in long term strategic investing. That said, we have simultaneously dramatically increased our long-term institutional ownership which fluctuates between about 9% and 16% of the Company. The vast majority of the reported institutional positions are NOT included within our NOBO lists. We welcome these forward-looking institutional shareholders who we suspect are investing for the long-term potential.
Another interesting trend we've noticed that seems to fly in the face of logic, is that during those several days of the year when we've had manic trading - days when we have traded more than 10 million to over 50 million shares in a single day - the actual turnover in our NOBO list is generally not more than a fraction of what you might expect. In other words, on those days when we've traded over 40 million shares in a day, we might only witness a couple hundred thousand shares actually changing hands in our NOBO list. It has always made us wonder "where are all those shares coming from?" And to this day, we have been unable to answer that question.
I want to say to all of our Lexaria shareholders, old and new, that the management team at Lexaria truly appreciates your support; that we will always work our hardest to try to achieve positive developments and increase the value of your company. No senior executive with Lexaria has sold LEXX stock and we remain absolutely committed to the success of this great company. Sometimes, we cannot explain our market activity which simply defies logic. But that won't stop us from remaining focused on our strategy, and on creating value for you over the longer term.
Judging from the increased number of shareholders as well as the increased quantity of shares owned, on average, more of you would agree with our plans and progress than disagree. Currently, we estimate that about 12,000 shareholders collectively own all of our 5.9 million shares.
Capital Markets
2021 began for Lexaria with a new listing on the Nasdaq Capital Markets which was the culmination of year-earlier plans to seek out stronger sources of capital and provide more liquidity for our shareholders. Nasdaq-reported volume for LEXX during 2021 is over 168,000,000 shares - an astonishing number given the fact that the Company only has about 5,900,000 shares issued and outstanding. Notwithstanding the seemingly "impossibly high" volume, one of our objectives for 2021 was to offer shareholders increased liquidity: given the fact that our split-adjusted volume for 2020 was only 1,320,660 shares, this 127-fold increase in volume certainly achieved that goal.
Another goal for 2021 was to escape what had earlier been a perpetual "hamster wheel" of corporate finance activities. In previous times the Company had generally struggled in locating sufficient working capital to pursue its objectives as rapidly and effectively as it wanted to. That era ended in January 2021 when we raised US$11 million in gross proceeds associated with our Nasdaq up-listing. That financing was budgeted to provide two years of working capital to aggressively accelerate our R&D plans, representing the highest R&D spend Lexaria had ever achieved.
We also raised approximately $4 million in July when some of the existing warrants that had been issued in January were exercised. That additional capital added to our working capital runway, giving us more time to advance our business plans. We are happy to report that our corporate spending for 2021 was on target and on budget in all material respects - we have carefully balanced our objectives with our abilities. Without raising another penny in capital we expect our existing resources to be sufficient until mid-year of 2023. Lexaria's management has long demonstrated financial prudence and clever methods to extract the most value possible from each dollar raised and we will continue to do so.
As we look towards 2022 and 2023, Lexaria is also taking steps to ensure we have multiple choices in how we fund the Company. Some details must remain undisclosed for now and will be revealed at the appropriate times, but in other respects we clearly are trying to position the Company for non-dilutive injections of capital through strategic partners and other commercial relationships.
Stock Performance
One area where Lexaria management is not at all satisfied, is with the Company's valuation and thus our stock price. During a year of exciting scientific progress and undisputed applied R&D advancement bringing us, we believe, closer than ever before to commercial relationships and success, the value of our company did not increase during the year. Indeed, during the early winter of 2021/22, our valuation declined to the lowest of the year which seems to defy logic considering our significant R&D progress.
We experienced inexplicably manic trading days in May, July and December when a quantity of shares equal to many times more than 100% of all our outstanding shares traded in a single trading day and, despite intraday price spikes reaching 52-week highs, there was little net change in value by the end of the day. While we cannot explain what seems at times as manipulative trading activity, rest assured that Lexaria and certain key shareholders have filed formal investigatory requests with the SEC, the Nasdaq, and FINRA; asking these regulators to step in and protect Lexaria shareholders. We will continue to monitor our market and seek every path and recourse available to us to protect your interests which are closely aligned with the Company's interests.
That said, it was a very difficult year across the broad biotech sectors, with most biotech companies losing value during the year. The SPDR biotech ETF, XBI, started the year at $139 and finished 2021 at $112: a decline of 19.5%. Genomic biotech ETF ARKG declined even more, from $91 to $62, dropping nearly 32%. And the Nasdaq biotech ETF, IBB, managed only a small gain of 2% on the year, after a year-end rally brought it out of the red. Regardless, it was a difficult year to earn a positive return in the biotech industry.
LEXX opened for trading on the Nasdaq near $4.25 and closed it's first day of trading near $4.50 before spending most of the year between $5.00 and $7.00. But we experienced a slide in valuation during the final six weeks of the year, along with an increase in reporting stock shorting, to end the year at a disappointing $4.03.
In part to address this, we recently approved the largest marketing plan in the Company's history, designed to ensure the public is aware of Lexaria's achievements in 2022; and, we have three major applied R&D studies that will launch and complete this year as well as many smaller programs. We expect that, if we have positive results, these study results could be enough to bring important industry relationships that could see us one big step closer to commercialization and revenues - obviously, this is tightly connected to our capital markets strategy in that we hope to take important steps towards commercial relationships in the year to come.
We will also be pursuing a pre- Investigational New Drug ("IND") meeting with the Food and Drug Administration ("FDA") for our most advanced drug product candidate, DehydraTECH-Cannabidiol ("CBD") for the treatment of hypertension and heart disease, in early 2022, and hope to complete our subsequent IND filing and obtain FDA approval to commence registered clinical testing during the year. That is only the start of our FDA-related plans; we will have more to discuss as time goes by. Our R&D plans are detailed in other areas of this letter, but they are an important ingredient within our plans for capital markets and value creation.
Research & Development
The results from our R&D can never be known in advance, but the initiation and progression of our R&D programs are within our control, and this is the aspect of 2021 of which we are most proud. Remember, our R&D efforts to date have largely been conducted to reduce or remove risks and to establish whether DehydraTECH is capable of being applied in broad use within the pharmaceutical industry. Meaningful national or international implementation of DehydraTECH is unrealistic prior to full knowledge and understanding of its limitations and capabilities. Lexaria has been very brazen and transparent conducting these studies in full public view compared to many pharmaceutical companies that keep these sorts of investigations hidden within their walls and undisclosed. Every positive result we generate is one more step towards removing risks associated with regulation and commercialization; eventually, with enough positive data, that formula will tilt in our direction.
Our R&D focus for 2021 was to investigate DehydraTECH CBD for possible hypertension and heart disease applications; to further our knowledge of DehydraTECH nicotine as a replacement for damaging and deadly lung-based absorption methods; and to learn whether DehydraTECH would be compatible with antiviral drugs. We were successful in each of these primary areas of investigation.
Hypertension and Heart Disease.
Our biggest area of investigation in 2021 was CBD for hypertension and heart disease, and we enjoyed a very successful year in this regard. Our first human study of the year, HYPER-H21-1, confirmed and built upon results we obtained in our 2018 human clinical study. This year we evidenced that human blood pressure ("BP") dropped within minutes of swallowing DehydraTECH-CBD capsules, after just a single dose.
In our HYPER-H21-2 follow-up study which administered 3 doses of DehydraTECH-CBD and monitored the volunteers over a 24-hour period, we witnessed even more impressive results. At selected times during the 24-hour study, volunteers with mild to moderate hypertension averaged as much as a 20 mmHg (i.e., 23%) decrease in BP relative to placebo. This is a large response after a single day of dosing and we were very encouraged by these results. For many existing prescribed blood pressure medications, similar decreases in BP are only apparent after many days or even weeks of dosing. Over the 24-hour ambulatory monitoring period, volunteers averaged a significant reduction of 7.0% in systolic pressure with DehydraTECH-CBD relative to placebo (p < 0.001).
In this same study, we also demonstrated with statistical significance for the first time that DehydraTECH-CBD reduced arterial stiffness in our human volunteers. Arterial stiffness is a strong predictor of many aspects of human diseaseand reducing it could lead to additional FDA regulatory findings as well as assist Lexaria in its goals of attracting a corporate pharmaceutical partner. Arterial stiffness is measured through pulse wave velocity ("PWV") evaluation, and it has been estimated that a 1.0 m/s increase in PWV accounts for a 15% increase in cardiovascular and all-case mortality.
This was one of our most important achievements of the year, which we feel evidences sufficient improvement in BP reduction to justify our ambitions within the regulated drug markets. In large part because our de-risking studies have been so positive, we formally announced our intention to pursue an IND filing with the FDA specifically for the purpose of developing DehydraTECH-CBD as a prospective registered treatment for hypertension and heart disease. That process is underway and will commence with a pre-IND meeting with the FDA in order to define our path to IND filing thereafter, and we expect to have an update within the next several months.
Beginning in April 2022 or possibly sooner, we expect to begin dosing in our largest ever hypertension study that will evaluate our DehydraTECH-CBD in 60 volunteers over a six-week study duration. This should be a large enough group to obtain reliable, statistically significant data and a long enough study duration to witness the true power of DehydraTECH-CBD in modulating BP, while observing if there are any serious adverse effects over a chronic dosing regimen period. If this study is successful, we feel strongly that it will be highly supportive of our IND filing plan, and we will have a clear path towards designs of Phase I and even potentially Phase II FDA-registered clinical studies thereunder. Assuming there are no major delays either in study execution or evaluation, we expect full results from this study sometime in Q3, 2022.
Nicotine.
In November 2021 we issued study results that demonstrated yet another first for Lexaria: in an animal study we evidenced that DehydraTECH was effective in promoting superior nicotine drug delivery characteristics in sublingual/buccal (oral) tissue.
DehydraTECH was originally developed to promote more efficient delivery of fat-soluble drugs across the intestinal wall, so our findings demonstrating its effectiveness also in enhancing sublingual/buccal (oral) absorption greatly enhances the value proposition for our technology for the oral nicotine products sector. This nicotine study was noteworthy not just because we delivered much higher levels of nicotine, more quickly; but also because we made this breakthrough utilizing buccal tissues of the mouth, gums and throat instead of through our traditional delivery through the intestines.
This was yet another opportunity demonstrating the versatility of DehydraTECH to work effectively in different parts of the body and opens doors to drug delivery through products such as lozenges, sub-lingual tablets, and other forms of non-swallowed oral products.
Because of this important new discovery for DehydraTECH, we are launching a very similar study in humans expected to start soon. If we can demonstrate similar performance of nicotine absorption through the sublingual/buccal tissue in humans, we feel that DehydraTECH will have advanced another big step in the direction of commercial applicability.
Antiviral.
Another area of significant advancement in 2021 was with antiviral drugs. We successfully demonstrated, for the first time ever, that DehydraTECH enhances delivery characteristics of certain antiviral drugs. This opens an entirely new class of drugs for investigation of applicability with DehydraTECH and helps to open long term business strategies that go beyond our core competencies with cannabinoids and nicotine. To date we have evidenced that DehydraTECH improves the delivery of each of the following drugs into animal bloodstreams: darunavir, efavirenz, remdesivir, ebastine, and colchicine; all of which are known to possess various antiviral properties.
We commissioned a study to evaluate DehydraTECH with the live SARS-CoV-2 virus. This study was performed using a primate cell line, VERO-E6, and conducted by a leading independent, US biosafety level 3 testing laboratory that delivers critical services to government and commercial customers. We learned that both remdesivir and ebastine (known to have poor aqueous solubility and compromised intestinal absorption and bioavailability when administered orally) processed with DehydraTECH were effective at inhibiting the COVID-19 SARS-CoV-2 virus using an in vitro screening assay in infected cells in Lexaria study VIRAL-C21-3.
We haven't confirmed much additional antiviral work in 2022 - this is not due to a lack of interest but instead simply because we are maximizing our financial and human resources towards other pursuits in 2022 that we feel are closer to commerciality. We are very encouraged by our antiviral progress in 2021, however, and we feel that it has demonstrated the tremendous flexibility of DehydraTECH to be applicable across a breadth of drug classes worthy of further investigation.
Seizure Disorders.
Beyond our key advancements described above, we also indicated in 2021 our intention to pursue expanded development opportunities for our DehydraTECH-CBD program. Lexaria is a global leader in conducting CBD research and we have completed more work in this field than many companies 20 times our size. Before the Spring of 2022 we expect to launch a complex animal study evaluating DehydraTECH-CBD as a potential treatment to inhibit seizure activity in animals. This, of course, is the same field where the former GW Pharmaceuticals had so much success and was able to achieve FDA certification for its version of CBD medication (Epidiolex®) in treating certain pediatric seizure disorders. Epidiolex® is thought to be currently generating about $160 million in quarterly revenue and continues to grow rapidly.
Although Epidiolex® enjoys regulatory exclusivity for its noted medical conditions, some of its exclusivity provisions are nearing expiration. We believe, therefore, that studies like those Lexaria is pursuing can help Lexaria attract commercial relationships and represent opportunities for DehydraTECH to enter commercial markets far more quickly than is generally thought. Accordingly, we have also announced our intention to pursue efficacy modelling through 2022 with our DehydraTECH-CBD in animals for other possible therapeutic indications of interest including dementia, rheumatoid diseases and diabetes.
Other R&D Advancements.
Throughout 2021, Lexaria also increasingly worked with DehydraTECH 2.0 formulations that we anticipate utilizing mostly within the FDA-regulated drug industry and that demonstrate our growing ability to optimize the "engine" that makes our drug delivery so fast and abundant. During 2021 we reached new heights of performance, delivering 2,178% and even 2,708% more CBD into animal blood than standard CBD industry formulations, in independent testing. Not only does this showcase the performance capabilities of DehydraTECH, it also casts a harsh eye on the woefully poor performance of many standard CBD industry practices that actually deliver as little as 2% of the labelled CBD into the bloodstream. That's abysmal and Lexaria challenges the industry to do better.
We also learned that DehydraTECH was remarkably stable in consumer beverages and that one year after production, bottled consumer beverages contained a remarkable 93.4% potency of CBD. We also showed less than 1% variability of CBD potency within the beverage, a concept of critical importance when delivering drugs in an aqueous solution. Beverages containing cannabinoids often need to be shaken prior to use and tend to degrade over time, sometimes dramatically. There have been reports of beverages sold that contain only a tiny fraction of the cannabinoids listed on the product label, whether due to inadequate manufacturing techniques or time decay. Lexaria's data demonstrate exceptional effectiveness in integrating CBD into Ready-to-Drink ("RTD") beverages in a stable and homogenously distributed manner over time without the need for physical mixing or agitation before consumption.
In 2018 we hired a third-party lab that evidenced that DehydraTECH was also capable of enhancing the delivery of cannabinoids across human epithelium ("skin"). While we have not yet pursued skin-based delivery markets, we have good early evidence of effectiveness. This is yet another area we are actively working on as it could open additional commercial pathways both in the regulated drug industry as well as with consumer goods. We have several prospective commercial clients that are indicating their desire to introduce their new skin-based products utilizing DehydraTECH to the market in Q2 or Q3 this year.
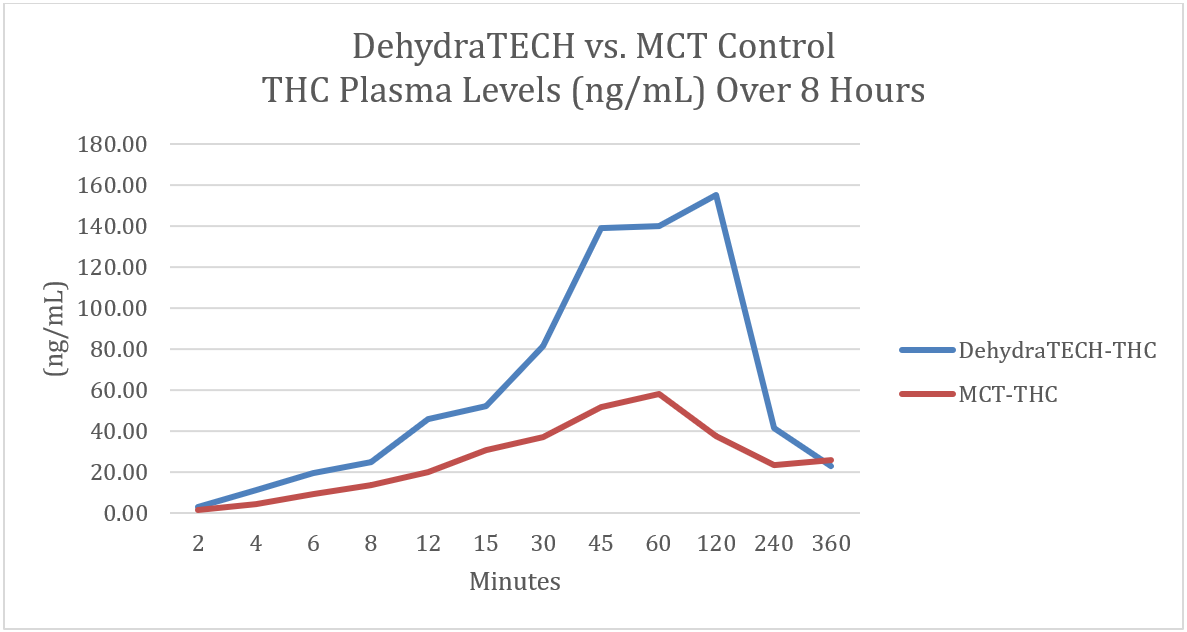
In a tetrahydrocannabinol THC pharmacokinetic ("PK") study we also demonstrated that DehydraTECH-processed THC was three times faster (15 minutes vs. 45 minutes) to deliver the same amount of THC into the bloodstream than generic THC. The DehydraTECH-processed THC also reached maximum blood saturation levels 2.5 times higher than the generic THC. Rapidity of action is particularly desired by customers of psychotropic drugs.
R&D Summary.
January 2021 really marked the birth of our modern version of Lexaria. During 2021, we completed R&D and validating work equal to or greater than all the combined amount previously completed since 2018! We have conducted studies across broad areas of interest, but also concentrated in specific areas where we have had supportive data. This is leading to substantial data sets that are increasingly supportive of discussions with potential commercial partners, as well as supporting our formal regulatory pathways.
During 2022 we are planning to enter the FDA IND pathway for DehydraTECH-CBD for hypertension as our first drug application. Lexaria has generated a lot of positive data both in animals and humans evidencing no significant adverse effects with DehydraTECH-CBD, and we do not expect that to change whether the health indication we are pursuing is hypertension, arterial stiffness, seizure disorders, or any other CBD-related application. If we continue to evidence safety with DehydraTECH-CBD, then our ability to pursue other health indications utilizing DehydraTECH-CBD might increase significantly if we provide evidence of effectiveness in any of those other areas of investigation.
We are launching three major studies in the March-April period: the 6-week human hypertension study (CBD); the animal seizure study (CBD); and the human sublingual/buccal tissue study (nicotine). Results from these studies are expected by early Summer to Fall. We will also be conducting a number of smaller studies throughout the year and will announce those when appropriate. All of our 2022 work is funded through existing resources, so we have no need to raise capital to complete this work.
Please make note: the COVID-19 pandemic is not over, and we hope you will grant us a little patience if any of our R&D investigations experience any delays in volunteer recruitment, or study execution. We do our best, but delays are possible.
The goal of our three major studies is to generate sufficient data to support either regulated IND-type applications; or to stimulate corporate partnering within their appropriate market sectors. We are optimistic of positive results - noting that science can be unpredictable - and, if so, expect 2022 to be our most exciting year ever!
Multiple Shots on Net in Huge Markets
Lexaria is a drug delivery technology company. Lexaria is not a CBD company, nor a nicotine company, nor an antiviral drug company. We do not define ourselves by any single molecule that seems to work with our DehydraTECH process, even though that molecule might represent years of opportunities and growth for the Company. CBD is obviously our "lead candidate" at this time and it has several possible routes towards success for us.
We are currently pursuing or investigating several large market opportunities, the smallest of which is currently generating over $10 billion in annual revenue. In many of these markets, growth is expected to be significant over the next several years. The Lexaria management team is trying to ensure that we have multiple paths to success: if any one single area of investigation were to fail, we want to ensure we still have other opportunities to exploit.
Size | Future Size | |||
| DehydraTECH Markets | US $bn | Year | US $bn | Year |
| Tobacco | 786.1 | 2022 | 908.3 | 2026 |
| Nicotine Replacement | 59.8 | 2022 | 147.9 | 2028 |
| CBD | 4.1 | 2022 | 111.8 | 2030 |
| Cardiovascular Drugs | 96.1 | 2022 | 107.8 | 2025 |
| Antivirals | 55.6 | 2022 | 66.7 | 2025 |
| Epilepsy | 10.6 | 2021 | 16.6 | 2031 |
| Human Hormones | 5.4 | 2022 | 13.0 | 2026 |
| PDE5 Inhibitors | 4.9 | 2022 | 6.0 | 2025 |
| Vitamin D3 | 1.2 | 2022 | 1.7 | 2026 |
Commercial Results
I'm often asked about well understood "normal" business metrics such as revenues, numbers of clients, and other similar aspects of our business. We had our highest ever revenues in 2021 of over $720,000 which is more than double that from one year earlier; and we generated a gross profit of $547,000 on this revenue, demonstrating the high quality of our revenue streams which are mostly associated with licensing of DehydraTECH. That said, we reported a big decline in revenues in our quarter ended November 30, 2021 due to some clients postponing additional orders.
Notwithstanding, we don't place a lot of relevance, yet, on our revenue numbers. That might seem counterintuitive, but upon reflection most people agree that is a reasonable comment. For the most part, our revenues to date are "proof of concept" or early-stage revenues: they show that we are able to gain and retain commercial clients willing to pay to use our technology, and that our DehydraTECH technology is available in the marketplace. But, given the early-stage nature of many of our clients, it is nearly impossible to build any type of reliable revenue model. Their revenues are expected to fluctuate widely, and as a result ours likely will too.
Lexaria's current clients are small companies anxious to establish themselves. They have worked hard - and we are delighted for them and their successful distribution in over 7,000 stores across America, including many well-recognized chains such as Hudson News, Albertson's, Safeway, and more. We expect our revenues will generally grow within these business segments, but they will not grow every quarter as challenges of various sorts are encountered.
And while important, these current revenue streams are not Lexaria's main area of business focus. We are focused on larger national and international applications for DehydraTECH, primarily but not entirely in the pharmaceutical sector. These are our areas of interest that we expect will provide significant revenue streams over time; but they have not yet begun.
The reason we spend so much time and resources on validating R&D programs, is to make our technology more attractive for large national and international consumer products companies and pharmaceutical companies. We know we have to provide copious amounts of data to satisfy the needs of the largest companies in their industries, and that these companies are naturally risk averse. We also expect that, if we do have success with these larger organizations, the commercial ramifications for Lexaria will be profound.
For example, many national or international corporations do not participate in new business segments unless they can be assured of multi-billion revenue potential. For large companies with sales of $10 billion or $30 billion per year, this makes sense. And given that Lexaria's business model is to out-license our technology in exchange for royalties, you can see very quickly how every single percentage point of royalty on each $1 billion in revenue, amounts to $10,000,000 per year in highly profitable revenue to Lexaria. For example, a 3% royalty rate on $4 billion would be $120,000,000 in revenue; or an 8% royalty on $2 billion would be $160,000,000 in revenue.
Furthermore, any out-licensing transactions Lexaria achieves with pharmaceutical companies may also be accompanied by significant, potentially large multi-million dollar staged development milestone fees that are customary in pharmaceutical licensing deals. These fees can at times be received years before a product ever reaches the market, or even regardless of whether a product ever reaches the market. Lexaria expects licensing fees to significantly complement its royalty revenue generation and also allow it to realize commercial revenue generation well in advance of when royalty revenues start to flow from actual product sales under these licensing transactions.
Thus, while we are grateful for our smaller clients and will continue to support those we have and work to gather more of them, a single larger client would quickly eclipse many years of growth of our smallest clients. Given that there are many regulated drugs that each enjoy annual revenue of more than $1 billion, you intuitively understand why we are positioning Lexaria for larger revenue streams with these longer-term plans.
Intellectual Property
Lexaria was awarded five new patents granted during 2021, bringing our total to 23 granted worldwide. New patents were awarded for the first time in India, Japan, and Mexico, continuing our efforts to receive intellectual property protection across wider areas of the world. With existing patents also granted in the USA, the European Union and Australia, our IP portfolio has never been stronger.
Within the vast market in India, our technology was patented in 2021 for use withnon-carbonated beverages, carbonated beverages, colas, root beers, fruit-flavoured beverages, citrus-flavored beverages, fruit juices, fruit-containing beverages, vegetable juices, vegetable-containing beverages, teas, coffees, dairy beverages, protein-containing beverages, shakes, sports drinks, energy drinks, and flavored waters. A similar patent was granted to Lexaria this year in Japan.
We expect additional patents to be awarded in 2022 although we acknowledge that the biggest growth in DehydraTECH-related IP is likely already achieved and solidified behind our patent portfolio.
Summary
Management of Lexaria feels we are on track, on schedule, and on budget to deliver what we hope and expect will be great results in 2022. If we generate sufficient positive data in ANY of our three major studies this year, we feel that positivity could be sufficient to enable our "graduation" to either the next logical regulatory step, or to a commercial relationship with one or more major industry partners.
We will continue working to advance our shorter-term business interests and meaningfully increase the modest revenue streams currently being generated. But we caution against applying undue reliance on those operations at this early stage in the Company's life. If we do not deliver higher revenue numbers, that is not particularly relevant to our primary business focus. If we do deliver higher revenue numbers, let's celebrate!
Before 2022 ends, we expect to have at least one major regulatory advancement such as an IND program approval, or one major new industry partner. We believe either of those will support what could be significantly higher valuations. If we are able to achieve more than one of these advancements…..so much the better.
Hundreds of you know me. You know I can't tell the future or forecast things outside of my control. But you also know that I and the Lexaria team are dedicated, and we believe in the Company and in the potential we have to make the world a better place. Your support has been so helpful in assisting us to achieve our mutually beneficial goals. We continue down this path, together.
Chris Bunka
The Company is not making any express or implied claims that it has the ability to eliminate, cure or contain COVID-19 (or the SARS-CoV-2) virus.
About Lexaria Bioscience Corp.
Lexaria Bioscience Corp.'s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream by promoting more effective oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids and nicotine by 5-10x and, in some instances with cannabinoids by as much as 27x compared to standard industry formulations, reduce time of onset from 1 - 2 hours to minutes, and mask unwanted tastes; and is also being evaluated for orally administered antiviral drugs, non-steroidal anti-inflammatory drugs (NSAIDs), PDE5 inhibitors and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 23 patents granted and over 50 patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements which are not historical facts are forward-looking statements. The Company makes forward-looking public statements including but not limited to: its expected future financial position, results of operations, cash flows, financing plans, research and development, business strategy, products and services, competitive positions, growth opportunities, plans and objectives of management for future operations, including statements that include words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions that are forward-looking statements. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that other factors will not affect the accuracy of such forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval or rejection process and other factors which may be identified from time to time in the Company's public announcements and filings. There is no assurance that existing capital is sufficient for the Company's needs or that it will be able to raise additional capital. There is no assurance the Company will be capable of developing, marketing, licensing, or selling products containing cannabinoids, nicotine or any other active ingredient or drug. There is no assurance that any planned corporate activity, scientific research or study, business venture, technology licensing pursuit, patent application or allowance, consumer or scientific study, or any initiative will be pursued, or if pursued, will be successful. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. The Company is not obligated to update this article in whole or in part, and every reader should rely only on the Company's official filings with the Securities and Exchange Commission for more up to date information. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease; only the FDA can grant approval for registered drugs within the US.
INVESTOR CONTACT:
George Jurcic - Head of Investor Relations
[email protected]
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.
View source version on accesswire.com:
https://www.accesswire.com/685781/Lexaria-Provides-Annual-Letter-From-the-CEO